Introduction
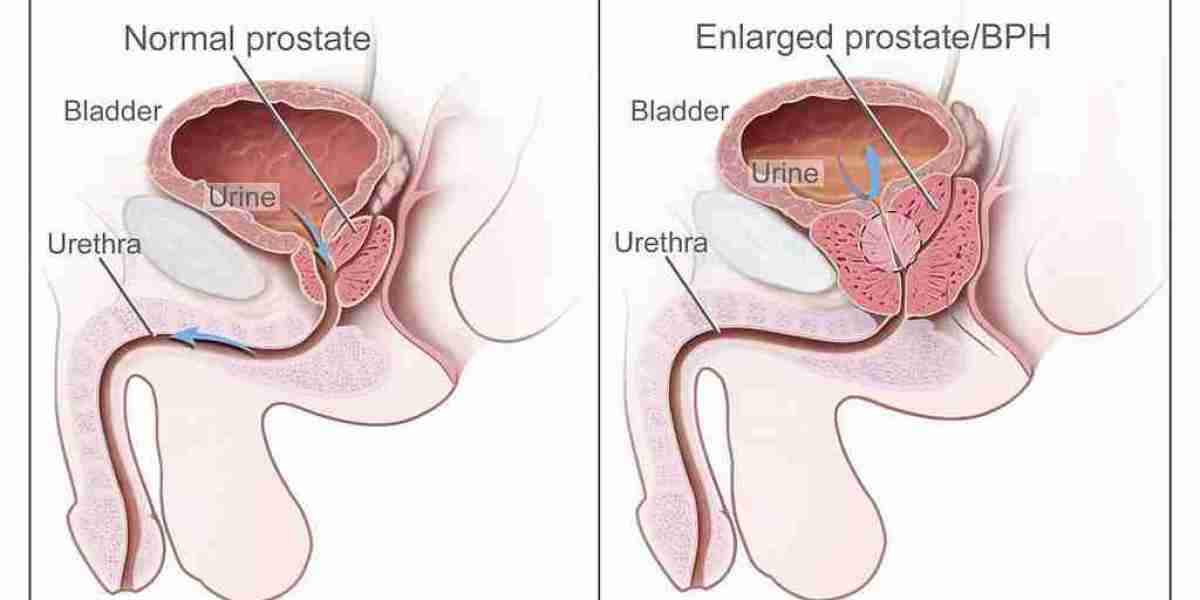
Prostate cancer continues to be one of the most significant health challenges for men worldwide, with non-metastatic castration-resistant prostate cancer (nmCRPC) representing a critical phase in disease progression. Traditionally, androgen deprivation therapy (ADT) has served as the frontline defense; however, its diminishing efficacy over time has spurred the need for innovative, precision-based treatments. Enter Nubeqa—a next-generation oral androgen receptor inhibitor that is rapidly redefining prostate cancer care. Developed by Bayer and Orion Corporation, Nubeqa’s active ingredient, darolutamide, has not only transformed the management of nmCRPC through its precision targeting but is now expanding its role to include metastatic hormone-sensitive prostate cancer (mHSPC). With robust clinical trial data backing its performance and a growing footprint in global markets, Nubeqa exemplifies the future of tailored oncology treatments.
For more in-depth insights on Nubeqa’s development and future potential, download the full report @ Nubeqa Market Report.
What is Nubeqa?
Nubeqa is an oral medication specifically designed for patients with nmCRPC who have progressed beyond the benefits of traditional ADT. The formulation centers around its active ingredient, darolutamide, which is engineered to block the effects of testosterone on cancer cells. By preventing testosterone from binding to androgen receptors, Nubeqa effectively slows down tumor progression and delays the onset of metastasis. This targeted approach has been recognized by regulatory bodies worldwide, with Nubeqa Approvals marking significant milestones in its clinical development. The drug’s innovative design minimizes its penetration of the blood-brain barrier, thereby reducing the neurological side effects that have plagued earlier generations of androgen receptor inhibitors. As a result, Nubeqa not only offers a potent alternative in nmCRPC treatment but also ensures a better quality of life for patients by maintaining cognitive function and overall well-being.
Nubeqa Mechanism of Action (MOA)
At the heart of Nubeqa’s success is its distinct mechanism of action. The Nubeqa active ingredient, darolutamide, exhibits high-affinity binding to androgen receptors, a critical step that prevents the usual cascade of testosterone-driven cancer growth. Unlike older androgen receptor inhibitors, darolutamide’s molecular structure is uniquely designed to avoid interaction with gamma-aminobutyric acid (GABA) receptors. This specificity is crucial in mitigating the risk of seizures and cognitive disturbances—a common drawback in prior treatments.
Preclinical studies have demonstrated that Nubeqa’s mechanism involves inhibiting the translocation of the androgen receptor to the nucleus, thereby interrupting the transcription of genes essential for tumor survival and proliferation. By effectively “switching off” these genetic signals, Nubeqa slows down tumor growth and prolongs metastasis-free survival. This innovative mechanism not only cements Nubeqa’s role in nmCRPC management but also lays the foundation for its potential application in mHSPC, as ongoing Nubeqa Clinical Trials explore broader therapeutic horizons. The precision of Nubeqa’s mechanism of action is a cornerstone of its clinical appeal, setting it apart in an increasingly competitive market.
For more detailed insights and the latest updates on Nubeqa, visit the Nubeqa Market update.
Clinical Efficacy and Safety
The clinical journey of Nubeqa is highlighted by its impressive efficacy and safety profile, as demonstrated in pivotal studies such as the ARAMIS trial. This landmark trial provided robust evidence that Nubeqa significantly extends metastasis-free survival by nearly two years compared to placebo. Patients treated with Nubeqa not only experienced delayed progression of pain and other debilitating symptoms but also maintained higher quality-of-life scores over extended periods. Such results underscore the drug’s dual promise: effective tumor control paired with an enhanced patient experience.
Safety considerations remain paramount in oncology, and Nubeqa’s design reflects this focus. The reduced likelihood of central nervous system side effects, owing to its limited blood-brain barrier penetration, marks a significant improvement over earlier treatments. Common side effects, including mild fatigue and manageable hypertension, are generally well tolerated, resulting in lower discontinuation rates. These factors have contributed to the drug’s inclusion in global treatment guidelines and have bolstered its reputation as a first-line option for nmCRPC. With each new Nubeqa Approval reinforcing its safety and efficacy, the drug continues to emerge as a trusted option among oncologists.
Nubeqa Cost and Accessibility
Despite its advanced technology and innovative formulation, Nubeqa is positioned as a cost-effective solution in the long term. Although the upfront cost reflects its status as a next-generation treatment, the ability of Nubeqa to delay expensive metastatic interventions offers significant economic advantages. By effectively postponing disease progression, the overall burden on healthcare systems is reduced, translating to both clinical and financial benefits.
Insurance coverage for Nubeqa is expanding, with policies increasingly covering its use in the United States, Europe, and Japan. Moreover, Bayer’s strategic partnerships with oncology networks and patient advocacy groups have enhanced its accessibility in emerging markets. Patient assistance programs further mitigate out-of-pocket expenses, ensuring that even those with limited resources can benefit from this breakthrough therapy. The expanding reach of Nubeqa, combined with its superior clinical profile, positions it as a leading contender in the fight against prostate cancer.
For further insights and detailed research on this breakthrough treatment, visit Nubeqa Insights.
Nubeqa Sales and Market Performance
The market performance of Nubeqa has been nothing short of remarkable. Nubeqa sales have surged since its launch, driven by its favorable safety profile, robust clinical data, and growing clinician confidence. In the United States, where high diagnosis rates and supportive reimbursement policies prevail, Nubeqa sales continue to dominate the nmCRPC space. European and Japanese markets are also witnessing an upward trend as awareness of the drug’s benefits spreads among healthcare providers.
Analysts at DelveInsight project that Nubeqa sales will experience robust growth over the next decade, reflecting its increasing adoption and the broadening of its therapeutic indications. The impressive track record in delaying disease progression has made Nubeqa a preferred option not only among patients but also within competitive market environments where alternatives like enzalutamide and apalutamide exist. As the drug’s applications expand into the mHSPC setting, further acceleration in Nubeqa sales is anticipated, reinforcing its strategic market position and long-term viability.
Future Outlook and Innovations
Looking ahead, the future for Nubeqa appears both promising and expansive. While its current approval for nmCRPC has already transformed the treatment landscape, ongoing clinical trials are evaluating its potential in the realm of metastatic hormone-sensitive prostate cancer (mHSPC). Early data from studies such as ARASENS suggest that Nubeqa may offer significant survival benefits in mHSPC, paving the way for its use as a cornerstone therapy in earlier stages of prostate cancer.
The research community is also exploring combination therapies involving Nubeqa. Studies integrating chemotherapy or PARP inhibitors aim to tackle resistant forms of the disease and further enhance patient outcomes. In parallel, advances in biomarker-driven care—such as AR-V7 testing—are expected to personalize treatment strategies, allowing clinicians to tailor Nubeqa use based on individual patient profiles. These developments not only promise to optimize therapeutic outcomes but also open new avenues for Nubeqa Clinical Trials, thereby extending its impact beyond nmCRPC.
Patent expirations in the future may invite biosimilar competition; however, the well-established efficacy and tolerability of Nubeqa are likely to safeguard its market position. The continuous stream of positive clinical outcomes and subsequent Nubeqa Approvals will further consolidate its status as a transformative therapy in prostate cancer management. With a focus on innovation and patient-centric care, Nubeqa is set to remain at the forefront of oncological advancements, embodying the shift from reactive treatment to proactive disease management.
For additional insights on Nubeqa’s transformative potential, please download the full Nubeqa report.
Conclusion
Nubeqa represents a paradigm shift in prostate cancer therapy, marking a significant stride from the treatment of nmCRPC to potential applications in mHSPC. With its active ingredient, darolutamide, Nubeqa’s precision mechanism of action has redefined the standard for androgen receptor inhibition, offering a treatment that not only delays metastasis but also preserves patient quality of life. The compelling results from pivotal clinical trials, such as the ARAMIS study, underscore its efficacy and safety, reinforcing its growing acceptance in global treatment guidelines.
The impressive market performance—evidenced by the surge in Nubeqa sales—coupled with expanding insurance coverage and accessibility measures, positions Nubeqa as a leader in the prostate cancer therapeutic arena. As ongoing research broadens its indications and refines combination strategies, Nubeqa is poised to deliver even greater clinical benefits. The continuous cycle of Nubeqa Approvals and positive clinical outcomes assures clinicians and patients alike that this innovative therapy is here to stay.
In summary, Nubeqa’s expanding therapeutic horizons signal a new era in prostate cancer management. Its unique approach to androgen receptor inhibition, combined with a favorable safety profile and robust market performance, sets a new benchmark for modern oncology. As the journey from nmCRPC to mHSPC unfolds, Nubeqa is not merely a treatment option—it is a testament to the power of precision medicine in transforming a once intractable disease into a manageable condition.
For those looking to explore this breakthrough treatment more, download the full Nubeqa Insights Report.
Read More
Prostate Cancer Market Insight, Epidemiology and Market Forecast
Metastatic Prostate Cancer Market Insight, Epidemiology And Market Forecast
Non-metastatic Prostate Cancer (nmPC) - Market Insight, Epidemiology and Market Forecast
Metastatic Castration-Resistant Prostate Cancer Market Insight, Epidemiology And Market Forecast
About DelveInsight
DelveInsight is a leading business Healthcare consultancy and market research firm specializing in life sciences. It assists pharmaceutical companies by offering comprehensive, end-to-end solutions to improve performance. Access all our healthcare and pharmaceutical market Competitive Intelligence Solutions.