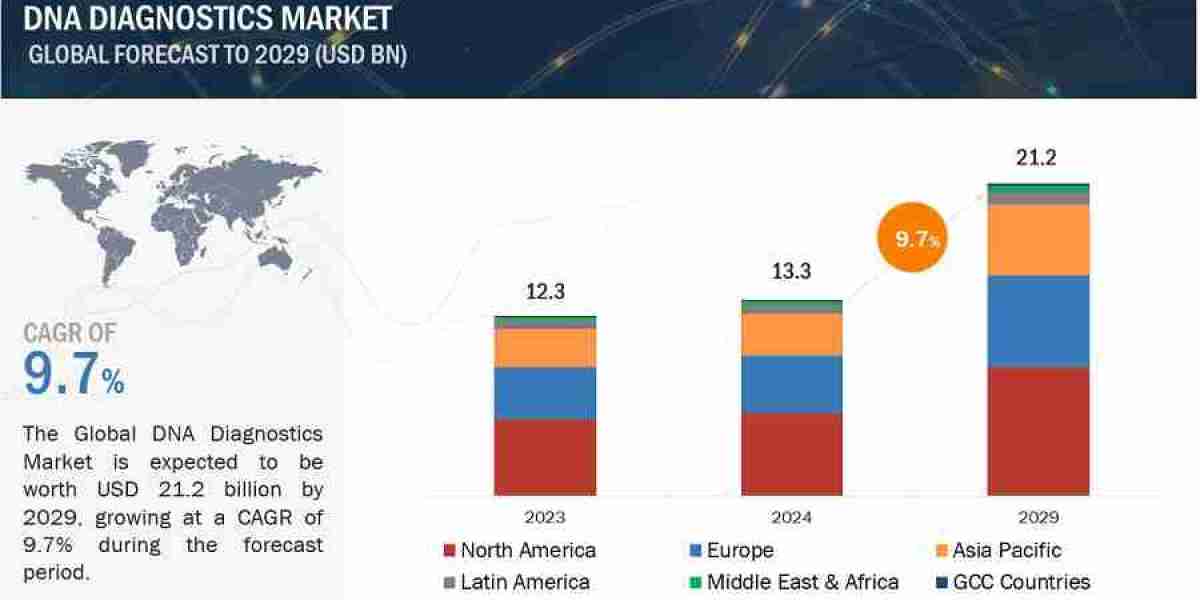
The global DNA Diagnostics Market valued at US$12.3 billion in 2023, is forecasted to grow at a robust CAGR of 9.7%, reaching US$13.3 billion in 2024 and an impressive US$21.2 billion by 2029. Growth in the market is primarily driven by the technological improvements in the DNA diagnostics industry and the growing need for personalized treatment and early disease diagnosis in developing nations. Also, the increased investments by healthcare-based companies are majorly contributing to growth of the market.
Key players:
The major players in this market are Illumina, Inc. (US), Danaher Corporation (US), F. Hoffmann-La Roche Ltd. (Switzerland), BIOMÉRIEUX (France), Hologic, Inc. (US), QIAGEN (Germany), Thermo Fisher Scientific Inc. (US), Abbott Laboratories (US), Siemens Healthineers AG (Germany), Myriad Genetics, Inc. (US), and Revvity Inc. (US). These players’ market leadership is due to their comprehensive product portfolios and expansive global footprint. These dominant market players have several advantages, including strong research and development budgets, strong marketing and distribution networks, and well-established brand recognition.
DNA Diagnostics Market Dynamics
DRIVER: Rising focus on R&D and increased funding by healthcare-based companies
Government initiatives, schemes, or funding activities that support and promote innovation and development provide growth opportunities in the market. These financial incentives and support systems enable companies/institutes to invest in R&D, infrastructure, and the production of advanced diagnostic technologies. By facilitating access to funds, manufacturers can push the boundaries of DNA diagnostics, leading to breakthrough innovations in disease detection, monitoring, and personalized medicine. For instance:
Some of the leading players operating in the market are investing significantly in research and development. For instance, in 2023, Abbott invested USD 2.74 billion, and F. Hoffmann-La Roche Ltd. invested USD 2.20 billion.
Restraint: Ethical and privacy concerns associated with DNA diagnosis
The development of each new genetic test brings significant challenges for medicine, public health, and social policy. These challenges include determining the appropriate circumstances for the test’s use, the methods for its implementation, and the applications of its results. After undergoing genetic testing, individuals have the right to privacy, which includes the ability to decide for themselves whether and with whom to share genetic information such as insurers, employers, educational institutions, employers, spouses and other family members, researchers, and social organizations.
A number of ethical concerns have emerged with respect to the privacy of the health data generated—for example, the possibility that giant software companies engaged in NGS data management may sell genome data. Another area of resistance is R&D on genetically modified organisms—an application of NGS. This is due to the possibility of environmental hazards arising from such experiments, risks to the food web, and issues concerning the prevalence of diseases and allergies, as well as contamination in animal test subjects. Such ethical issues serve to challenge the growth of DNA diagnostic applications.
Opportunity: Advancements in bioinformatics and artificial intelligence in DNA diagnostics
Advancements in bioinformatics and artificial intelligence (AI) have significantly impacted DNA diagnostics, leading to more efficient, accurate, and accessible testing methods. A major use of bioinformatics is the identification of genes in large DNA sequences. Prior to the advent of bioinformatics, the only methods available for locating genes along a chromosome were either isolating the DNA and studying it in a test tube (in vitro) or studying the gene’s behavior in the organism (in vivo). By utilizing a computer to analyze sequence data, bioinformatics enables experts to make educated estimates regarding the location of genes (in silico). A cancer patient’s prognosis is enhanced by early detection; however, early diagnosis may be challenging to accept. Technology has evolved with the use of DNA microarrays and proteomics studies for large-scale gene expression research, which has increased the use of bioinformatics tools.
However, AI and genetic engineering have opened new avenues for biotechnology and personalized medicine. Machine learning algorithms can analyze large-scale genetic sequence datasets, which can then be used to steer the development of more accurate and effective genome editing technologies by predicting probable off-target consequences. This is one way that AI helps predict and optimize genome editing methods like CRISPR-Cas9. These advancements are revolutionizing the landscape of DNA diagnostics, enabling more precise, timely, and personalized healthcare solutions.
To determine the current size of the DNA diagnostics market, this study engaged in four main activities. A comprehensive study was conducted using secondary research methods to gather data about the market, its parent market, and its peer markets. The next stage involved conducting primary research to confirm these conclusions, assumptions, and sizing with industry experts throughout the value chain. A combination of top-down and bottom-up methods was used to assess the overall market size. The market sizes of segments and subsegments were then estimated using data triangulation techniques and market breakdown.
The four steps involved in estimating the market size are
Collecting Secondary Data
Within the secondary data collection process, a range of secondary sources were reviewed so as to identify and gather data for this study, including regulatory bodies, databases (like D&B Hoovers, Bloomberg Business, and Factiva), white papers, certified publications, articles by well-known authors, annual reports, press releases, and investor presentations of companies.
Collecting Primary Data
During the primary research phase, a comprehensive approach was adopted, involving interviews with a diverse array of sources from both the supply and demand sides. These interviews aimed to gather qualitative and quantitative data essential for compiling this report. Primary sources primarily comprised industry experts spanning core and related sectors, as well as favored suppliers, manufacturers, distributors, service providers, technology innovators, and entities associated with all facets of this industry's value chain. In-depth interviews were meticulously conducted with a range of primary respondents, including key industry stakeholders, subject-matter authorities, C-level executives representing pivotal market players, and industry advisors. The objective was to obtain and authenticate critical qualitative and quantitative insights and to evaluate future potentialities comprehensively.
Market Size Estimation
All major product providers offering various products and services were identified at the global/regional level. Revenue mapping was done for the major players and was extrapolated to arrive at the global market value of each type of segment. The market value of the DNA diagnostics market was also split into various segments and subsegments at the region and country level based on:
- Offering mapping of various manufacturers for each type in the DNA diagnostics market at the regional and country-level
- Relative adoption pattern of each DNA diagnostics market among key application segments at the regional and/or country-level
- Detailed primary research to gather qualitative and quantitative information related to segments and subsegments at the regional and/or country level.
- Detailed secondary research to gauge the prevailing market trends at the regional and/or country level
Recent Developments of DNA Diagnostics Industry:
- In March 2024, F. Hoffmann-La Roche Ltd. (Switzerland) launched cobas Malaria test. The cobas Malaria test received approval from the US Food and Drug Administration (FDA) for use on the cobas 6800/8800 systems.
- In November 2023, Illumina Inc. (US) launched an assay, TruSight Oncology 500 ctDNA v2. This assay enables noninvasive comprehensive genomic profiling (CGP) of circulating tumor DNA (ctDNA) from blood when tissue testing is not available or to complement tissue-based testing.
- In December 2023, F. Hoffmann-La Roche Ltd. (Switzerland) partnered with Global Fund (Switzerland) to support low- and middle-income countries in strengthening critical diagnostics infrastructure.
Content Source:
https://www.marketsandmarkets.com/Market-Reports/dna-diagnostics-market-145577831.html
https://www.marketsandmarkets.com/PressReleases/dna-diagnostics.asp