The pharmaceutical inspection machines market is experiencing significant growth, driven by stringent regulatory requirements, technological advancements, and the increasing complexity of pharmaceutical products. As of 2024, the market is valued at approximately $0.9 billion and is projected to reach $1.4 billion by 2029, reflecting a compound annual growth rate (CAGR) of 7.6%.
Download PDF Brochure: https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=81453085
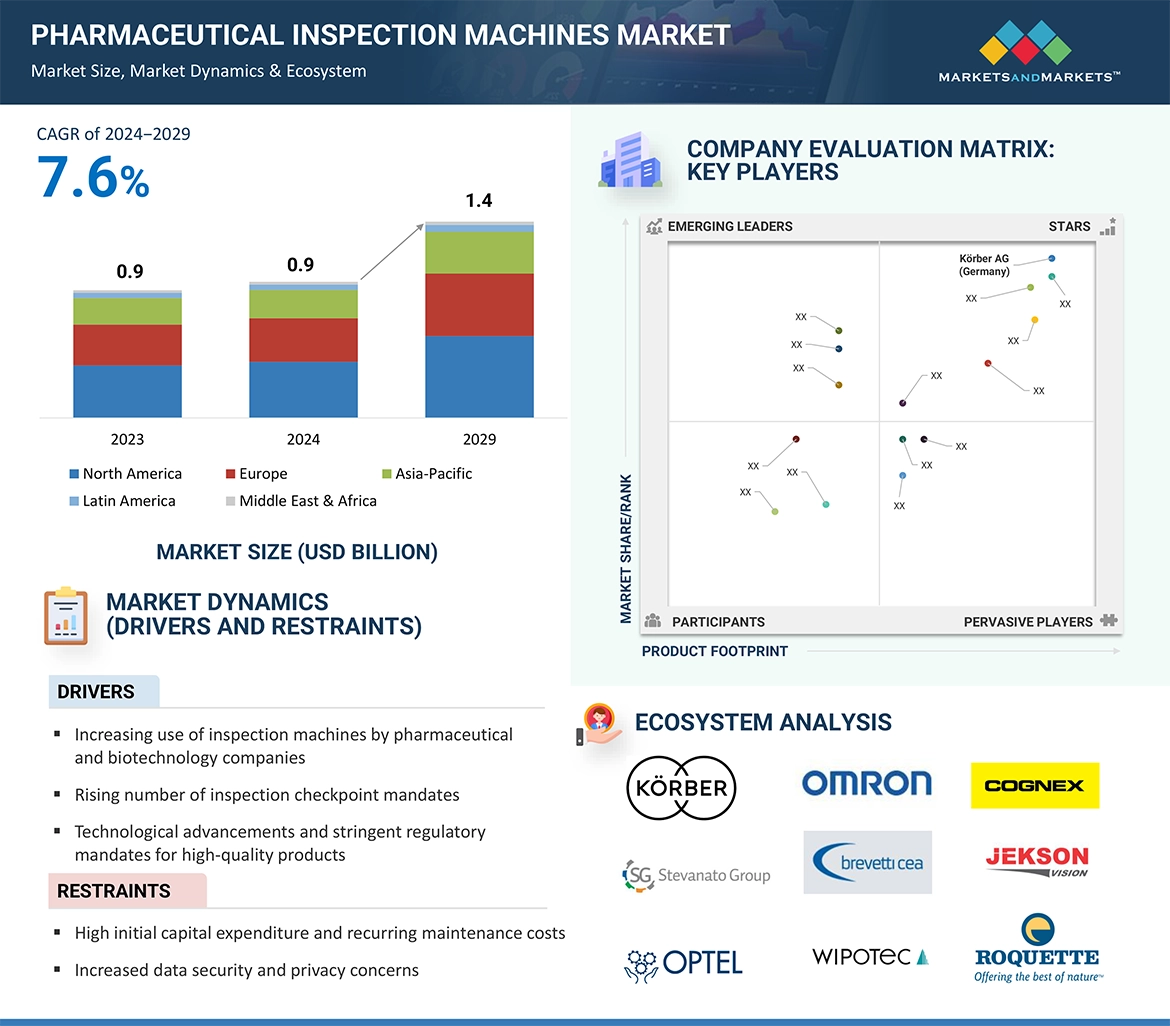
Key Market Drivers
- Stringent Regulatory Requirements: Regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) enforce strict guidelines to ensure pharmaceutical product safety and efficacy. Compliance with these regulations necessitates the adoption of advanced inspection machines capable of detecting contaminants and defects in pharmaceutical products.
- Technological Advancements: The integration of technologies such as artificial intelligence (AI), machine learning (ML), and the Internet of Things (IoT) into inspection systems has enhanced their accuracy, speed, and functionality. These innovations enable real-time monitoring and predictive maintenance, reducing downtime and improving overall efficiency.
- Increasing Pharmaceutical Outsourcing: The rise in pharmaceutical outsourcing, particularly in emerging economies, has led to a greater need for standardized inspection processes to ensure product quality across different manufacturing sites. This trend has bolstered the demand for inspection machines globally.
Market Segmentation
The pharmaceutical inspection machines market is segmented based on product type, application, and region:
- By Product Type:
- Vision Inspection Systems: Utilize cameras and image processing software to detect visual defects in products and packaging.
- X-Ray Inspection Systems: Employ X-ray technology to identify internal defects or contaminants not visible externally.
- Checkweighers: Ensure product weight consistency, crucial for dosage accuracy.
- Metal Detectors: Detect metal contaminants in products, ensuring safety and compliance.
- By Application:
- Ampoules & Vials: Inspection for cracks, impurities, and fill levels.
- Blisters: Ensure seal integrity and correct product placement.
- Syringes: Check for needle defects, fill volume, and particulate matter.
- Tablets & Capsules: Assess for size, shape, color consistency, and coating integrity.
Regional Insights
North America holds a significant share of the pharmaceutical inspection machines market, attributed to advanced healthcare infrastructure, stringent regulatory frameworks, and substantial investment in research and development. The Asia-Pacific region is anticipated to exhibit the highest growth rate, driven by increasing pharmaceutical manufacturing activities, rising healthcare expenditures, and growing adoption of advanced technologies.
Request Sample Report: https://www.marketsandmarkets.com/requestsampleNew.asp?id=81453085
Challenges
Despite the positive outlook, the market faces challenges such as high initial investment costs, which can be prohibitive for small and medium-sized enterprises. Additionally, complexities in integrating new inspection technologies into existing production lines can hinder adoption.
Growth Opportunities
- Adoption of Automation: The shift towards automation in pharmaceutical manufacturing presents opportunities for the deployment of advanced inspection machines that offer higher throughput and consistent accuracy.
- Emerging Markets: Expanding pharmaceutical industries in emerging economies provide a fertile ground for market growth, especially as these regions adopt stricter quality control measures.
- Technological Integration: The ongoing integration of AI, ML, and IoT into inspection systems is expected to drive innovation, offering enhanced capabilities such as predictive analytics and real-time quality control.
Conclusion
The pharmaceutical inspection machines market is poised for substantial growth, driven by regulatory imperatives, technological advancements, and the expanding global pharmaceutical industry. By addressing current challenges and leveraging emerging opportunities, industry stakeholders can enhance product quality, ensure compliance, and achieve sustained success in this dynamic market.







